Much faster clinical timelines: Deployment in ~6 months saving years in pipeline
Much lower capital and operational investment: Less than half the traditional budget with much smaller equipment footprint and minimized site construction
Flexible re-deployable clinical manufacturing platform
Clinical trial now at the speed of R&D
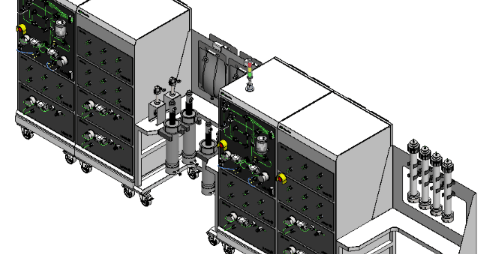
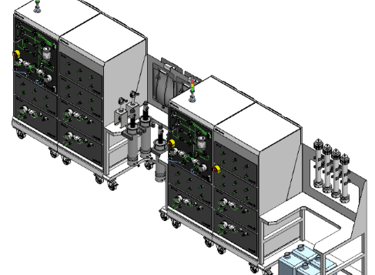
A turn-key downstream bioprocess configurable using M++ modules for any DSP unit operation
Flexible from 500 to 4000L clinical manufacturing capacity
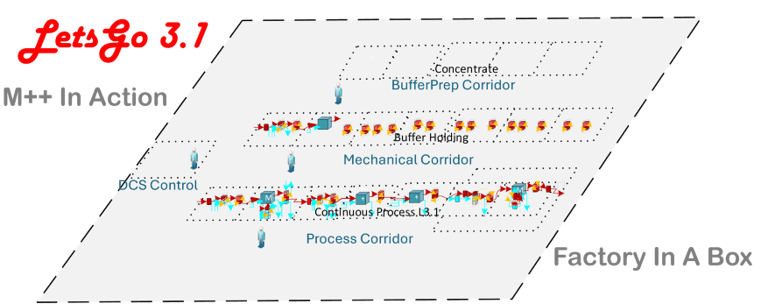
Continuous processing with constant feed-in and constant product-out
Continuous Inline-Dilution Buffer Preparation
Small footprint/Minimized construction
True one-click operation - no manual handling, no operator variability
Fully enclosed, cGMP, automated system with DCS control and S88 batch management
No CIP utilities required
Up to 50% less affinity (Protein A) resin and smaller systems and columns
Copy-and-paste tech transfer - Ready to be scaled up to full commercial production
No additional process development - A continuous process based on your own optimization
Easy parallel expansion
Delivered In A Truck
The bottleneck in drug development has always been the clinical manufacturing, the wait for making enough drug material for clinical trial. Traditional clinical manufacturing production takes 2-3 years to build and consumes enormous capital and operational resources. Every additional day of wait in the pipeline costs millions of dollars. LetsGo 3.1 is a clinical manufacturing solution that replaces permanent infrastructure with re-deployable platform with speed, flexibility, and control.