Purification Made Simple
Fully Scalable Clinical Manufacturing At the Speed Of IND
Blog post description.
1/22/20261 min read
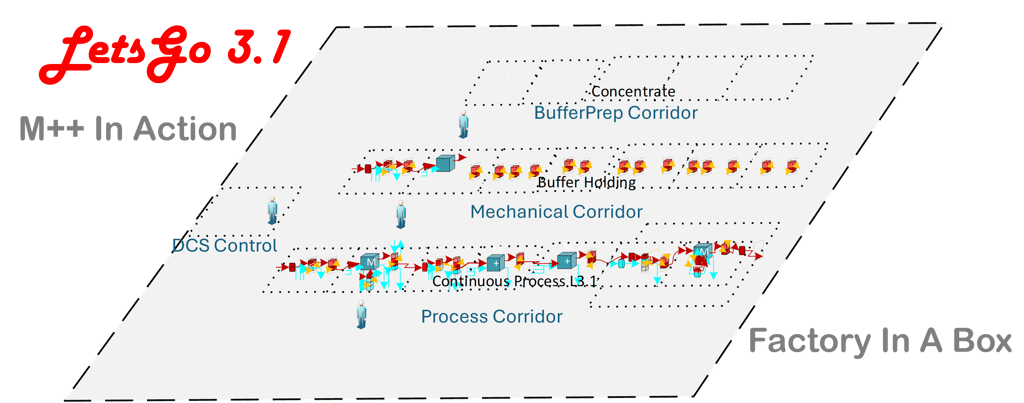
Clinical Manufacturing at the Speed of Development
In modern drug development, time is capital. Yet clinical manufacturing remains one of the largest sources of delay. Two to three years spent building infrastructure means programs wait, teams stall, and capital is tied up before proof of success.
LetsGo 3.1 directly addresses this mismatch.
A New Operating Model for Clinical Manufacturing
LetsGo 3.1 is not a facility. It is a re-deployable clinical manufacturing platform that replaces permanent infrastructure with a standardized, automated system.
Built on M++ modular downstream processing, the platform supports any DSP unit operation and implements continuous processing directly from existing batch processes. No additional process development is required to move into continuous operation.
This enables faster readiness without compromising process integrity.
What Faster Really Means
Deploying LetsGo 3.1 typically takes around six months. That difference matters.
For development teams, it means:
Clinical material availability aligned with trial readiness
Fewer idle periods waiting on manufacturing capacity
Earlier data generation and faster decision-making
For leadership teams, it means years removed from the pipeline and meaningful reduction in time-related financial exposure.
Engineering Simplicity, Operational Consistency
From an MSAT and manufacturing execution perspective, the platform offers standardized hardware, control, and automation across deployments. One-click operation removes variability tied to manual steps. Fully enclosed processing simplifies environmental control and validation.
Affinity resin usage can be reduced by up to 50% through smaller columns and more efficient system design, lowering consumable costs while supporting continuous operation.
Designed for Growth And Transfer
Capacity expansion does not require rebuilding facilities. Parallel systems can be deployed to support additional programs or increased demand. The same platform scales forward to commercial production by copy-n-paste both hardware and software, reducing late-stage tech transfer risk.
This allows organizations to commit capital and resources when programs justify it, not years earlier.
Manufacturing That No Longer Sets the Pace
LetsGo 3.1 removes clinical manufacturing as the limiting factor in development. It enables speed, flexibility, and control in a single deployable system.
LetsGo 3.1: M++ In Action is available now.
If you are responsible for CMC strategy, MSAT execution, or clinical manufacturing delivery, this is a conversation worth having.
