Purification Made Simple
Continuous Processing With Much Smaller Footprint
Blog post description.
1/22/20262 min read
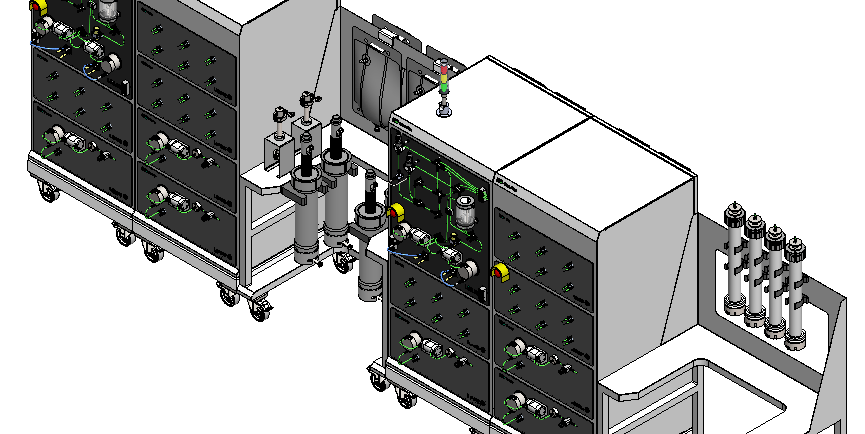
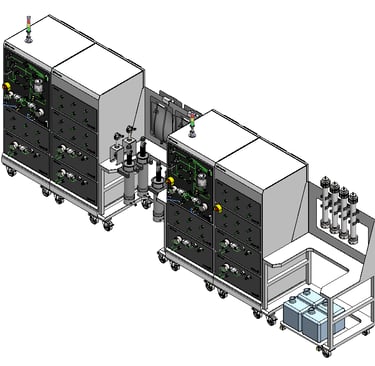
Removing the Clinical Manufacturing Bottleneck
For decades, the slowest part of drug development has not been discovery or clinical design. It has been clinical manufacturing. The wait to produce enough drug substance for trials routinely stretches timelines by years.
Traditional clinical manufacturing infrastructure takes two to three years to design, build, qualify, and validate. It consumes enormous capital and operational resources long before clinical outcomes are known. Every additional day waiting for material delays trials and can cost millions across the development pipeline.
This is the bottleneck LetsGo 3.1 is designed to remove.
From Permanent Infrastructure to Deployable Manufacturing
LetsGo 3.1 replaces facility-centric manufacturing with a re-deployable downstream platform. Instead of building permanent infrastructure, organizations can deploy a complete clinical manufacturing system directly to site.
The platform is delivered as a fully enclosed, automated, cGMP-compliant production line built on M++ modular architecture. It supports continuous downstream processing with constant feed-in and constant product-out, allowing stable operation without batch-level interruptions.
Deployment timelines are reduced from years to approximately six months, saving significant time in the clinical pipeline.
Speed Without Sacrificing Control
Speed alone is not enough. Clinical manufacturing must remain controlled, compliant, and repeatable.
LetsGo 3.1 achieves this through:
Fully automated operation with DCS control and S88 batch management
One-click execution with no manual handling and minimal operator variability
Continuous inline-dilution buffer preparation
A fully enclosed system that simplifies validation and compliance
Residence Time Based Mini-Batch collection for releasing and tracking
Complete history of startup, steady-state run, and shutdown
System challenge tests
The result is faster readiness without introducing operational risk.
Capital Efficiency That Matches Clinical Reality
Traditional manufacturing forces early, irreversible capital commitments. LetsGo 3.1 shifts that model.
With a smaller equipment footprint, minimized site construction, and no CIP utility requirements, total capital and operational investment is reduced to less than half of traditional clinical manufacturing budgets. Capacity can be added when needed, rather than forecast years in advance.
Clinical trials can now move at the speed of R&D, not at the pace of facility construction.
A Platform Built for Change
LetsGo 3.1 is flexible by design. Capacity ranges from 500 L to 4000 L, and expansion is achieved through easy parallel deployment. The same system architecture supports copy-and-paste technology transfer and is ready to scale toward commercial production without additional process development.
This makes manufacturing an adaptable resource rather than a fixed constraint.
