Streamlining Tech Transfer - The Role of Modular Benchtop Platforms
5/7/20252 min read
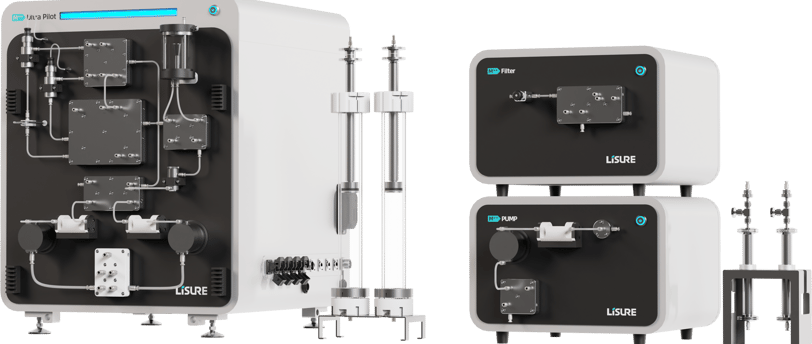
Introduction to M++ Technology Transfer Platform
The pharmaceutical industry continually seeks methods to enhance efficiency and reliability in drug development. A significant innovation in this area is the modular, multi-functional technology transfer platform. Designed with cGMP industrial flow path and DCS/S88 batch control capabilities, these systems represent a considerable step forward in streamlining technology transfer through drug development pipeline.
Understanding Key Features
The benchtop multi-functional modular platform is specifically tailored to facilitate the transfer of technology across different phases of drug development pipeline. This holistic approach integrates various functionalities within a single platform, enabling effective transitions from one process stage to another. With an emphasis on process intensification, this platform aids in reducing time and cost while maintaining regulatory compliance.
Advantages of a One-Stop Technology Transfer Solution
By combining multiple process configurations into a single platform, the technology transfer becomes significantly more efficient. This one-stop solution minimizes the complexity often seen in traditional setups, where multiple technologies and systems must interface with one another. The streamlined nature of the modular platform helps to mitigate risks commonly associated with the drug development pipeline and provide better support to CMC submission.
The incorporation of cGMP design principles ensures that the platform adheres to industry best practices, further enhancing its reliability and effectiveness. This feature also aids companies in ensuring compliance with regulatory guidelines, a crucial aspect of pharmaceutical manufacturing.
Moreover, the DCS/S88 batch control solutions integrated into the platform allow for precise monitoring and control over processes. This capability is vital for maintaining consistency and quality control throughout the clinical manufacturing stages. As the pharmaceutical landscape grows more complex, embracing such advanced technology becomes essential for companies aiming to stay competitive.
Conclusion
The evolution of drug development is intrinsically linked to advancements in technology, and modular multi-functional platforms are at the forefront of this change. By adopting a technology transfer solution that encapsulates various processes and industrial hardware and software standards within a single system, pharmaceutical manufacturers can not only enhance operational efficiency but also improve their overall development pipeline. As we look to the future, the integration of such platforms will likely shape the next generation of tech transfer and CMC data management throughout drug development pipeline.
