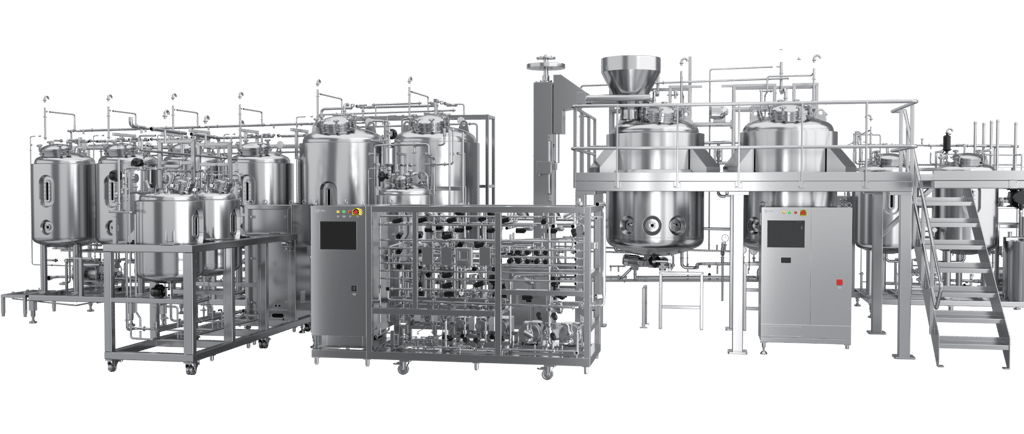
Connected and Continuous Bioprocessing
How Continuous Buffer Management Systems (CBMS) Are Redefining Bioprocessing
Blog post description.
6/1/20253 min read
Introduction
The biopharmaceutical industry is entering a new era of efficiency, automation, and scalability. At the heart of this transformation lies a critical but often underappreciated operation—buffer preparation. For decades, large tank farms dominated this domain, but as facilities expand and demand intensifies, these traditional systems are revealing serious limitations. Enter the Continuous Buffer Management System (CBMS)—an innovation poised to revolutionize how buffer solutions are prepared, managed, and delivered in GMP production environments.
From Tank Farms to Agile Systems
Historically, buffer tank farms functioned like chemical laboratories on steroids: large vessels premixed specific buffer formulations, which were then stored and cleaned after each use. While functional, this method became unsustainable as scales increased—some production facilities now require tanks as large as 30,000 liters. The cleaning, validation, and space required for such operations create substantial cost and logistical burdens.
Inline dilution skids brought the first major shift about two decades ago. By blending concentrated buffers with WFI at the point of use, they minimized vessel size and improved process agility. But these systems remained closely tied to individual unit operations.
The Emergence of CBMS
CBMS takes process intensification to the next level. Instead of preparing buffers for individual processes, the CBMS produces and delivers buffer solutions for entire buffer preparation areas. It replaces massive hold vessels with relay tanks—compact containers typically one-tenth the size—that are filled via high-precision inline mixing modules. Think of the shift as going from DVDs (tank farms) to streaming services (CBMS): more flexible, on-demand, and connected.
Key hardware includes:
Concentrate tanks holding acids, bases, and salts
Inline mixing modules with real-time pH/conductivity monitoring
Relay tanks for downstream staging
A complex valve matrix controlling fluid distribution
Economic and Operational Impact
One of the most compelling reasons to adopt CBMS is the cost savings. Replacing large vessels with smaller relay tanks reduces capital expenditures—some projects report savings of 30–60%. Smaller CIP/SIP operations further cut operational costs, often by 20–50%. Importantly, the reduced GMP footprint opens up valuable facility space for other critical functions.
Beyond dollars, CBMS improves batch documentation and data integrity. Powered by industrial DCS platforms, it offers automated recipe-driven control, precise monitoring, and minimal manual intervention. In fact, buffer filling is governed by liquid-level and buffer-priority logic, eliminating the need for tight coupling with downstream equipment.
Overcoming Implementation Challenges
Deploying a CBMS is not plug-and-play. Challenges include:
System complexity with multiple integrated modules
Flow rate precision under dynamic pressure conditions
Harmonized process control for buffer delivery and tracking
Facility-specific layout customization
Valve matrix management with over 100 valves in some cases
These complexities have made CBMS development a tall order for many OEMs. But when addressed through a standardized, GAMP-validated OEM solution, the system becomes robust, scalable, and adaptable.
Real-World Validation
To date, CBMS has been implemented in roughly 20 production lines across various global biopharma facilities. Projects range in scale and incorporate both stainless steel and single-use technologies. Buffer delivery quality—confirmed by pH and conductivity data—matches or exceeds that of traditional tank systems. Just as critically, users report a smoother tech transfer process, with reduced risk of pressure-related chromatographic disturbances during buffer switches.
Strategic Benefits for Modern Biomanufacturing
CBMS aligns tightly with broader industry trends in digital transformation and Industry 4.0. Its automation-centric design, batch recipe flexibility, and data transparency make it an ideal platform for smart manufacturing environments.
Whether designing a greenfield facility or retrofitting a legacy plant, CBMS offers unmatched versatility, compliance readiness, and process intensification capabilities. It decouples buffer preparation from process operation, allowing biopharma manufacturers to scale with agility and maintain consistent quality in a cost-effective manner.
The Continuous Buffer Management System marks a new frontier in downstream bioprocessing. By combining real-time inline mixing, compact relay tanks, and intelligent process control, it reduces costs, improves traceability, and supports the drive toward continuous biomanufacturing. For companies seeking to streamline their operations and prepare for future demands, CBMS is no longer an option—it’s a strategic imperative.