Purification Made Simple
Downstream Process Intensification (PI): The Impact of Inline Buffer Exchange SSTFF, RTF Chromatography, and Tankless TFF
5/21/20252 min read
New Paradigm in Downstream Purification
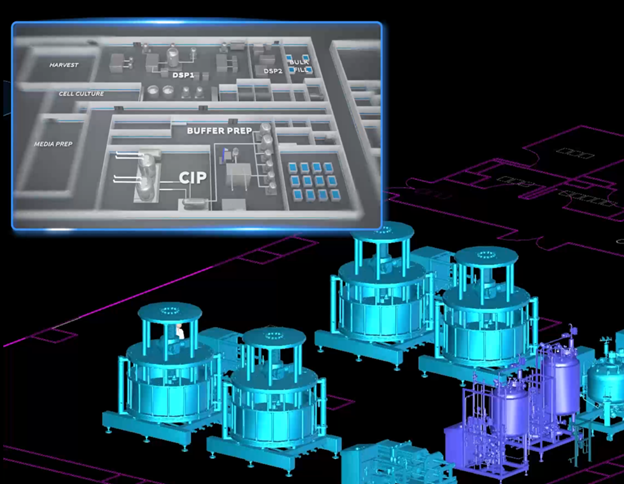
The advancements in biopharmaceutical production have led to the development of innovative methods for downstream purification. Recent breakthroughs involve the integration of inline buffer exchange using SSTFF (steady-state tangential flow filtration), Recirculating Tangential Flow (RTF) chromatography, and TMP/CF independent tankless TFF (tangential flow filtration) processes. These technologies enable the handling of particulates and cells in an inline fashion, direct loading of unconditioned feed to chromatography, and immediate connection of TFF after chromatography, signifying a monumental shift in the way protein purification, particularly monoclonal antibody (mAb) purification, can be performed.
Process Intensification (PI) For Purification Process
Traditionally, a typical mAb downstream purification process could entail up to nine distinct steps. This not only requires substantially more time but also utilizes a larger footprint for equipment and facility space. However, by employing the groundbreaking techniques of inline buffer exchange, RTF chromatography for particle/cell handling, and tankless TFF, this complex nine-step process can be condensed into just two to three connected batches. Based on current industrial standard (https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/bit.28641), this technical advancement qualifies as a Level 3 process intensification which the industry is still struggling to achieve. Such a transformation is significant as it directly addresses some of the long-standing challenges in the biopharmaceutical industry, including connecting two steps without a surge tank in between and software challenge in orchestrating process control in two or more separate steps.
The Advantages of Reduced Processing Time and Footprint
Level 3 process intensification directly addresses challenges in cGMP space limitation, lengthy process cycle time, and overall low efficiency. First and foremost, shorter cycle times can lead to greater productivity and immediately reduction on production cost in pharmaceutical manufacturing. Faster processing allows for a quicker turnaround, enabling companies to meet rising global demands for mAbs and other protein drugs without compromising on quality. Secondly, a condensed process footprint reduces the operational costs associated with both manpower and resource utilization, positively impacting both operational agility and overhead cost allocation in production cost.
With a smaller equipment footprint, manufacturers can optimize their facility layout, freeing up valuable space that can be repurposed for other critical operations or expansions. This efficient use of space is not just a matter of convenience, it can significantly lower capital expenditures and enhance overall business profitability.
The Future of Protein and mAb Purification
The shift to inline buffer exchange and tankless TFF is a game changer for the pharmaceutical industry. By being able to simplify the mAb downstream purification process into fewer steps, companies can improve efficiency, reduce costs, and maximize their production capabilities just as the ongoing process intensification effort in the industry promises. As technology continues to evolve, the focus will undoubtedly remain on enhancing these critical methods further, paving the way for even more advancements in bioprocessing. The combination of these novel approaches marks a significant leap toward intensifying protein therapeutics such as mAb production, making it more sustainable and more capable of meeting global healthcare needs particularly by adopting the level 3.1 process intensification (PI), the highest level in process intensification where there is only a continuous steady-state process with constant raw material incoming flow and constant product outgoing flow.